Effects of High or Low Estrogen in Men
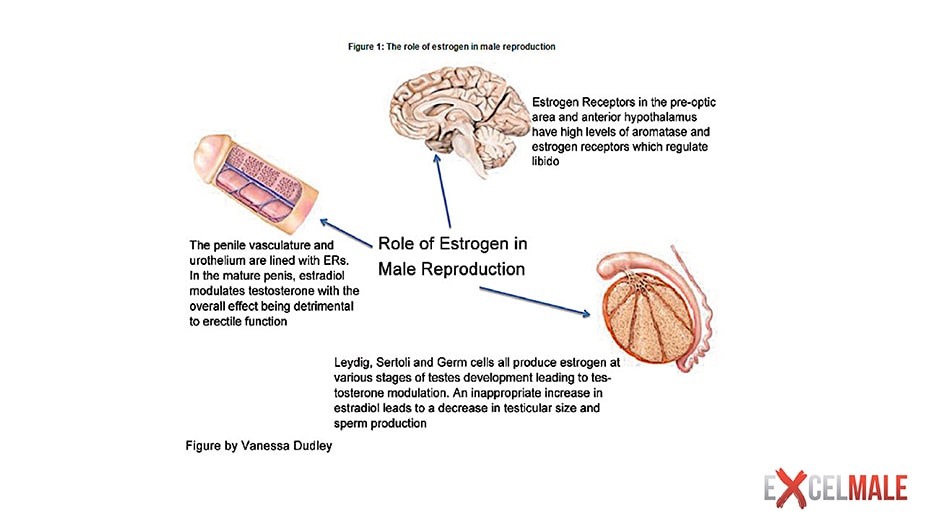
Table of Contents
- Why is Estradiol Important in Men?
- THE ROLE ON ESTROGEN IN MEN:
- THE EFFECT OF ESTRADIOL ON FAT MASS, SEX DRIVE, AND ERECTILE FUNCTION
- EFFECT OF ESTRADIOL ON MORTALITY IN MEN:
- THE EFFECT OF ESTRADIOL ON BONE DENSITY IN MEN:
- THE EFFECT OF HIGH ESTRADIOL ON LIBIDO IN MEN ON TESTOSTERONE THERAPY
- High Estrogen in Men After Injectable Testosterone Therapy TRT
- THE EFFECT OF LOW ESTRADIOL ON BONE DENSITY IN MEN:
- How to Get a sensitive estradiol test
Why is Estradiol Important in Men?
Testosterone is the precursor hormone for estradiol. Estradiol is a hormone more abundant in women than men that is produced by the aromatization of testosterone in liver, fat and other cells. Nature created it for a reason. It has been shown to be responsible for healthy bone density but its role in men's sex drive, body composition and other variables is source of great debate. One thing is certain: High estradiol blood levels can cause gynecomastia in the presence of low testosterone.
WATCH LECTURE ABOUT ESTRADIOL IN MEN BY CLICKING HERE
When the HPT (Hypothalamic-Pituitary-Testicular) hormonal axis senses that testosterone or estradiol is high, it automatically decreases or shuts down testosterone production.
Many anti-aging or men's health clinics prescribe anastrozole, a blocker of estradiol production, to men who start testosterone replacement (TRT). Higher estradiol blood levels not only can cause breast tissue growth (gynecomastia) but also water retention (edema). Some people speculate that high estradiol can also lead to erectile dysfunction but no scientific papers have been published on this subject. Since higher testosterone blood levels can originate higher estradiol levels, the belief is that using anastrozole will prevent breast tissue growth and erectile dysfunction by lowering any potential increase in estradiol. However, we have no data on how high is too high when it comes to this hormone in men. Some even speculate that low testosterone-to-estradiol ratios may be more closely correlated to gynecomastia and erectile problems than estradiol alone.
The truth about these speculations is starting to emerge but we still do not have enough data to say what the upper value of the optimal range of estradiol really is. We have a lot of evidence about the lower side of the optimal range since it has been found that estradiol blood levels below 20 pg/ml can increase bone loss in men. A recently published study also nicely demonstrated that low estradiol can be associated with higher fat mass and lower sexual function in men. So, be very careful when a clinic wants to put you on this drug without first justifying its use.
Another concerning fact is that many clinics may be using the wrong estradiol test that may be over-estimating the levels of this hormone in men by using the old estradiol test (ECLIA based.) A sensitive estradiol test (LC/MS assay) more accurately measures estradiol in men in comparison to the regular test that costs less (ECLIA immunoassay based). This sensitive test does not have interference from inflammation markers like CRP that result in overestimation of estradiol in men using the non-sensitive ECLIA test. Ensure that your doctor is using the right test!
Fortunately, most men on TRT do not develop gynecomastia even without using anastrozole (gynecomastia is more common in bodybuilders who may use high doses of testosterone and/or anabolic steroids, however). Those that have gynecomastia at TRT doses (100-200 mg of injectable testosterone or 5-10 grams of testosterone gel per day) may be genetically predisposed to having more aromatase activity or have liver dysfunction. Treating all men who start TRT with anastrozole from baseline may be counterproductive since this may lower estradiol to very low levels. Some physicians monitor estradiol blood levels after 6-8 weeks of TRT initiation (with no anastrozole) using the sensitive estradiol test to determine if anastrozole use is warranted. Doses range from 0.25 mg per week to some clinics using excessive doses of 1 mg three times per week. After 4-6 weeks on anastrozole, its dose can be adjusted to ensure than estradiol is not under 20 pg/ml. Fortunately, many men on TRT do not need anastrozole at all.
So we wait for more studies that will clarify the role and optimal ranges of estradiol. Here are a few studies that we already have available based on the role investigated.
THE ROLE ON ESTROGEN IN MEN:
From: The Decline of Androgen Levels in Elderly Men and Its Clinical and Therapeutic Implications
There is a rapidly growing body of evidence that a number of physiological actions of testosterone in men are mediated by the ERs (estrogen receptors) after its biotransformation by the aromatase P450 inhibitors in the tissues . Documented estrogen-mediated actions of testosterone in men include a role in the feedback regulation of LH , a role in the regulation of skeletal homeostasis , as well as a role in lipid metabolism and cardiovascular physiology ; among other possible estrogen actions in men, there are indications for a role in the brain and in spermatogenesis. These estrogenic actions in men can be exerted by blood-borne estrogens as well as through local aromatization of testosterone in, or in close vicinity of, the target cell. The expression of the CYP19 gene encoding the aromatase enzyme can be differentially regulated according to the tissue.
The conversion rate of testosterone to estradiol is around 0.2%. Up to 80% of plasma estradiol originates from aromatization of testosterone and androstenedione, mainly in subcutaneous fat and striated muscle, although aromatase activity is present in many other tissues, including bone and the brain; no more than 20% of estradiol in the circulation is secreted by the testes. Estradiol serum concentration in the adult male is around 20 to 30 pg/ml (70 to 110 pmol/liter), with a production rate of around 45 μg/d. Plasma estradiol is also bound to SHBG but with only half the affinity of testosterone. Total plasma estradiol levels in adult men do not vary significantly with age; indeed the decrease in precursor levels (i.e., testosterone and androstenedione) is compensated by an increase of fat mass and tissue aromatase activity with age. As a consequence of the age-associated increase in SHBG binding capacity, the serum concentrations of free estradiol and non-SHBG-bound or “bioavailable” estradiol do show a moderate age-associated decrease. It can be pointed out that estrogen serum levels in elderly males are higher than those in postmenopausal women.
THE EFFECT OF ESTRADIOL ON FAT MASS, SEX DRIVE, AND ERECTILE FUNCTION
There was a recently published groundbreaking study about the role of estradiol in men. No optimal ranges of estradiol were noted but low levels were associated with increased fat and decrease in sexual desire and erectile function compared to higher levels (the highest average estradiol was 35 pg/ml, unfortunately, so no conclusions can be made for levels above this).
Men had their hormones blocked by a gonadotropin-releasing hormone antagonist. All of them then received testosterone supplementation with Androgel. Half were also treated with anastrozole to block estradiol conversion from the testosterone.
All participants (Cohorts 1 and 2) received goserelin acetate (Zoladex, AstraZeneca), at a dose of 3.6 mg subcutaneously at weeks 0, 4, 8, and 12, to suppress endogenous gonadal steroids (testosterone and estradiol). All participants (Cohort 1 and 2) were then randomly assigned to receive 0 g (placebo), 1.25 g, 2.5 g, 5 g, or 10 g of a topical 1% testosterone gel (AndroGel, Abbott Laboratories) daily for 16 weeks. Participants in cohort 2 also received anastrozole (Arimidex, AstraZeneca) at a dose of 1 mg daily to block the aromatization of testosterone to estrogen. Participants were unaware of the study group assignments.
Findings:
Higher blood levels of testosterone decreased the percentage of body fat (P = 0.001), intra abdominal fat area (P = 0.021), and subcutaneous fat area (P = 0.029), and increased sexual desire (P = 0.045) and erectile function (P = 0.032).
Low blood level of estradiol was associated with significant increases in the percentage of body fat (P<0.001), subcutaneous fat area (P<0.001), and intra abdominal fat area (P = 0.002), and relative less improvement in sexual desire (P<0.001) and erectile function (P = 0.022). These findings provide additional evidence of an independent effect of estradiol on these variables.
"Our finding that estrogens have a fundamental role in the regulation of body fat and sexual function, coupled with evidence from prior studies of the crucial role of estrogen in bone metabolism, indicates that estrogen deficiency is largely responsible for some of the key consequences of male hypogonadism and suggests that measuring estradiol might be helpful in assessing the risk of sexual dysfunction, bone loss, or fat accumulation in men with hypogonadism. For example, in men with serum testosterone levels of 200 to 400 ng per deciliter, sexual desire scores decreased by 13% if estradiol levels were 10 pg per milliliter or more and by 31% if estradiol levels were below 10 pg per milliliter. "
Reference:
Gonadal Steroids and Body Composition, Strength, and Sexual Function in Men
N Engl J Med 2013;369:1011-22.
EFFECT OF ESTRADIOL ON MORTALITY IN MEN:
This study found that estradiol levels of < 21.80 pg/ml and > 30.11 pg/ml resulted in greater mortality in men.
Abstract
CONTEXT:
Androgen deficiency is common in men with chronic heart failure (HF) and is associated with increased morbidity and mortality. Estrogens are formed by the aromatization of androgens; therefore, abnormal estrogen metabolism would be anticipated in HF.
OBJECTIVE:
To examine the relationship between serum concentration of estradiol and mortality in men with chronic HF and reduced left ventricular ejection fraction (LVEF).
DESIGN, SETTING, AND PARTICIPANTS:
A prospective observational study at 2 tertiary cardiology centers (Wroclaw and Zabrze, Poland) of 501 men (mean [SD] age, 58 [12] years) with chronic HF, LVEF of 28% (SD, 8%), and New York Heart Association [NYHA] classes 1, 2, 3, and 4 of 52, 231, 181, and 37, respectively, who were recruited between January 1, 2002, and May 31, 2006. Cohort was divided into quintiles of serum estradiol
quintile 1, < 12.90 pg/mL;
quintile 2, 12.90-21.79 pg/mL;
quintile 3, 21.80-30.11 pg/mL;
quintile 4, 30.12-37.39 pg/mL;
and quintile 5, > or = 37.40 pg/mL.
Quintile 3 was considered prospectively as the reference group.
MAIN OUTCOME MEASURES:
Serum concentrations of estradiol and androgens (total testosterone and dehydroepiandrosterone sulfate [DHEA-S]) were measured using immunoassays.
RESULTS:
Among 501 men with chronic HF, 171 deaths (34%) occurred during the 3-year follow-up. Compared with quintile 3, men in the lowest and highest estradiol quintiles had increased mortality (adjusted hazard ratio [HR], 4.17; 95% confidence interval [CI], 2.33-7.45 and HR, 2.33; 95% CI, 1.30-4.18; respectively; P < .001). These 2 quintiles had different clinical characteristics (quintile 1: increased serum total testosterone, decreased serum DHEA-S, advanced NYHA class, impaired renal function, and decreased total fat tissue mass; and quintile 5: increased serum bilirubin and liver enzymes, and decreased serum sodium; all P < .05 vs quintile 3). For increasing estradiol quintiles, 3-year survival rates adjusted for clinical variables and androgens were 44.6% (95% CI, 24.4%-63.0%), 65.8% (95% CI, 47.3%-79.2%), 82.4% (95% CI, 69.4%-90.2%), 79.0% (95% CI, 65.5%-87.6%), and 63.6% (95% CI, 46.6%-76.5%); respectively (P < .001).
Reference:
Circulating estradiol and mortality in men with systolic chronic heart failure.
JAMA 2009 May 13;301(18):1892-901.
THE EFFECT OF ESTRADIOL ON BONE DENSITY IN MEN:
This study followed young and older men's testosterone and estradiol to see their impact on bone density. Estradiol below 11 pg/ml was associated with increased bone loss.
Abstract
Estrogen appears to play an important role in determining bone mineral density in men, but it remains unclear whether estrogen primarily determines peak bone mass or also affects bone loss in elderly men. Thus, we assessed longitudinal rates of change in bone mineral density in young (22–39 yr; n = 88) vs. elderly (60–90 yr; n = 130) men and related these to circulating total and bioavailable estrogen and testosterone levels. In young men bone mineral density increased significantly over 4 yr at the mid-radius and ulna and at the total hip (by 0.32–0.43%/yr), whereas it decreased in the elderly men at the forearm sites (by 0.49–0.66%/yr), but did not change at the total hip. The rate of increase in bone mineral density at the forearm sites in the young men was significantly correlated to serum total and bioavailable estradiol and estrone levels (r = 0.22–0.35), but not with total or bioavailable testosterone levels. In the elderly men the rates of bone loss at the forearm sites were most closely associated with serum bioavailable estradiol levels (r = 0.29–0.33) rather than bioavailable testosterone levels. Moreover, elderly men with bioavailable estradiol levels below the median [40 pmol/liter (11 pg/ml)] had significantly higher rates of bone loss and levels of bone resorption markers than men with bioavailable estradiol levels above 40 pmol/liter. These data thus indicate that estrogen plays a key role both in the acquisition of peak bone mass in young men and in bone loss in elderly men. Moreover, our findings suggest that age-related decreases in bioavailable estradiol levels to below 40 pmol/liter may well be the major cause of bone loss in elderly men. This subset of men is perhaps most likely to benefit from preventive therapy.
Reference:
Relationship of Serum Sex Steroid Levels to Longitudinal Changes in Bone Density in Young Versus Elderly Men.
The Journal of Clinical Endocrinology & Metabolism August 1, 2001 vol. 86 no. 83555-3561
THE EFFECT OF HIGH ESTRADIOL ON LIBIDO IN MEN ON TESTOSTERONE THERAPY
I have been warning men and clinics not to be so aggressive treating estradiol in men with the overuse of anastrozole. Here is a recent study was done in Houston.
Elevated serum estradiol is associated with increased libido in men receiving testosterone replacement therapy (TRT), according to researchers.
In a study of 423 men on TRT, Ranjith Ramasamy, MD, working with Larry Lipshultz, MD, at the Baylor College of Medicine in Houston, measured subjects' testosterone and estradiol levels and asked the men to rate the quality of their libido using a five-point Likert scale (1= terrible, 5 = excellent). The researchers categorized the men as having low or high testosterone (below or above 300 ng/dL, respectively) and low or high estradiol (below 5 and above 5 ng/dL (50 pg/mL), respectively).
Men with high serum testosterone levels reported significantly greater libido than men with low level and those with high serum estradiol levels had significantly greater libido than subjects with low levels. In all, 60.4% of men with both high testosterone and estradiol levels reported very good or excellent libido (score as 4 or 5) compared with 31.3% of participants with both low testosterone and estradiol levels, the researchers reported in European Urology (published online ahead of print). These results are expected to be presented at the American Urological Association annual meeting in Orlando.
High Estrogen in Men After Injectable Testosterone Therapy TRT
The Low T Experience
Robert S. Tan, Houston
Abstract
Testosterone replacement improves quality of life and is aromatized in men in adipose tissues to estrogen. Hyperestrogenism is believed to be harmful to male sexuality. This is a description of our experience of screening 34,016 men in the Low T Centers, of which approximately 50% were converted to treatment. Men were treated with injectable testosterone, and we have available data from 2009 to 2014. The data were extracted from our electronic health record (AdvancedMD) of 35 Low T Centers across the United States. In all, 7,215 (20.2%) out of the 34,016 patients had high estradiol levels defined as ≥42.6 pg/ml. Estradiol was measured using electro-chemiluminescence immunoassay. Of the patients who had high estradiol levels, the age distribution was as follows: 132/989 (13.3%) were older than 65 years, 3,753/16,955 (22.1%) were between 45 and 65 years; 2,968/15,857 (18.7%) were between 25 and 44 years, 7/215 (3.3%) were younger than 25 years. The difference between extreme age groups (<25 and ≥65) was statistically significant using a chi-square test (p = .013). The correlation coefficient of serum estradiol to age was .53,SD = 8.21. It was observed that practitioners used aromatase inhibitor and selective estrogen receptor modulator to treat symptoms of hyperestrogenism, irrespective of blood estradiol levels. Gynecomastia was rarely documented as a reason for the prescription. Our finding was that high estradiol levels were not associated with higher rates of low libido but established higher rates of documented low libido with those with normal or lower estradiol levels. The difference was statistically significant (p< .05).
THE EFFECT OF LOW ESTRADIOL ON BONE DENSITY IN MEN:
Low blood levels of estradiol are associated with brittle bones in old men: New study.
Serum Estradiol Levels are Inversely Associated with Cortical Porosity in Older Men.
J Clin Endocrinol Metab. 2014 Apr 2:jc20141319.
Abstract
Context: The key role of serum estradiol (E2) for bone health in men is well established. The effect of serum sex steroids on bone microstructure, measured by high-resolution peripheral quantitative computed tomography (HRpQCT), remains unknown in elderly men. Objective: To examine the associations between serum sex steroids and bone microstructural parameters in older men. Methods: Trabecular and cortical bone microstructure at the tibia was measured by HRpQCT in 440 men (mean 80 years of age) participating in the population-based MrOS Sweden cohort. Serum levels of E2 and testosterone (T) were analyzed with mass spectrometry and free E2 and free T levels were calculated using law-of-mass-action equations. Results: Age-adjusted models demonstrated that E2 and free E2 but not T or free T associated significantly inversely with cortical porosity. The associations between E2 and free E2 and cortical porosity remained significant after further adjustment for height, weight, physical activity, calcium intake and smoking. Models including both serum E2 and T demonstrated that E2 (standardized beta= -0.12, P<0.05) but not T associated independently with cortical porosity. A similar independent association was found for free E2 (standardized beta= -0.12, P<0.05) but not free T. Free E2 associated significantly with trabecular bone volume fraction in age-adjusted models but this association did not remain significant after further adjustment. Conclusions: Serum E2 levels associated inversely with cortical porosity in 80-year-old men. We propose that low serum E2 may reduce cortical bone strength, at least partly by increasing cortical porosity, and, thereby, increase fracture risk in older men.
Here is a more detailed discussion on estradiol in men: Click here
How to Get a sensitive estradiol test





